Researchers in Brazil, led by Alessandro Pasqualotto, have shown that a 10mg/Kg single dose of liposomal amphotericin B, followed by itraconazole is at least as effective as daily liposomal amphotericin B.
Clinical centres in Brazil screend 882 adult patients with HIV infection for disseminated histoplasmosis. From this sample population they randomised 118 people to be included in the study.
The majority of the original 822 patients were not included as they were found to not have histoplasmosis.
For those patients included in the trial, a single dose of 10mg/Kg of AmBisome was compared in a 1:1:1 ratio to two doses of AmBisome (10mg/Kg followed after 48 hours by 5mg/Kg) and to 3mg/Kg AmBisome daily for 14 days. All patients received 400mg of itraconazole from day 2 and continued for a year.
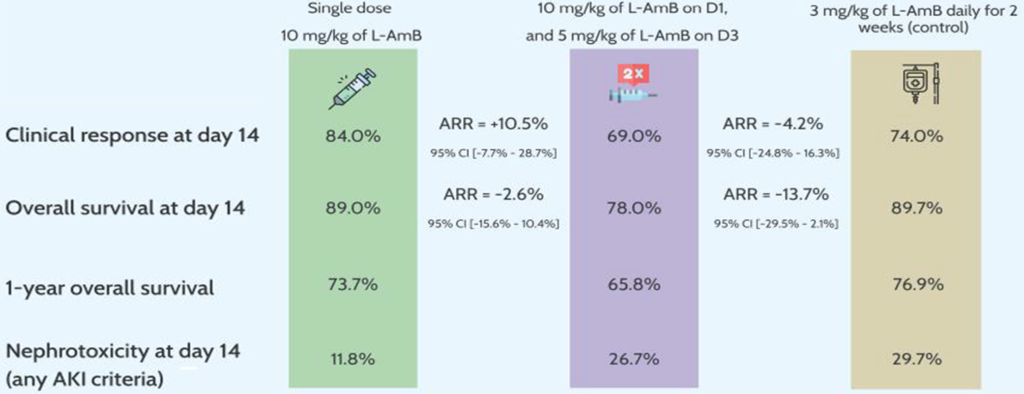
There was no difference in overall mortality, and the survival rates were very good for this disease – ~70% at 12 months. However clinical response was highest at day 14 in the single dose group at 84% and nephrotoxicity was lower at 11.8%, under half that seen in the other 2 groups.
Liposomal amphotericin B (and possibly specifically AmBisome) is probably superior to conventional amphotericin B based on a randomised study published in 2002. High, single dose liposomal amphotericin B is effective for visceral leishmaniasis and cryptococcal meningitis (the latter if combined with flucytosine and fluconazole).
These data should allow more patients with HIV to survive histoplasmosis. Questions remain over the optimal management of those with both histoplasmosis and tuberculosis, given the problem with itraconazole and rifampicin drug interactions. Patients who were diagnosed with tuberculosis were excluded from this study for this reason.
For more information on histoplasmosis, see our dedicated resource pages.
