There are currently no approved vaccines for fungal infections.
Vaccination against invasive fungal infections, like candidaemia or invasive pulmonary aspergillosis, could benefit patients who are (or will become) immunosuppressed (e.g. those receiving chemotherapy, transplants/haematopoietic stem cell transplants and people with HIV/AIDS). Unfortunately, a large proportion of these people would be unable to mount a normal immune response to a vaccine. As fungal infections are less common than bacterial ones, there is less incentive for large pharmaceutical companies to invest in their development.
- The Candida vaccine NDV-3A (NovaDigm Therapeutics) has shown promise in a Phase 1b/2a trial for reducing the frequency of episodes of recurrent vulvovaginal thrush (Edwards et al, 2018). This vaccine was previously known as PEV7 and was owned by Pevion.
- Pan-fungal vaccines based on BDG or heat-killed brewer’s yeast (Saccharomyces) have unfortunately not proceeded into human trials.
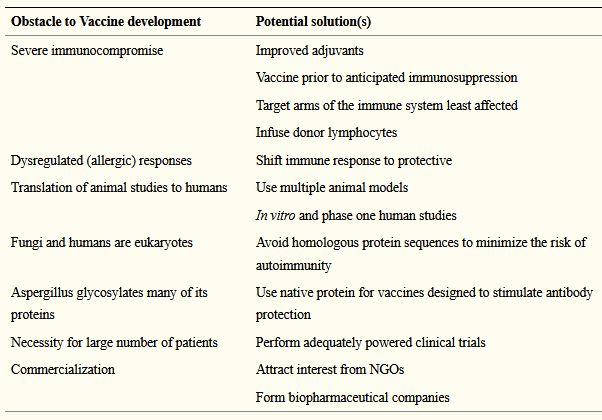
- Levitz (2017) provides a helpful table of suggestions for overcoming some of the main obstacles to development of an Aspergillus vaccine, and discusses the possible use of immunotherapy.
- For a comprehensive description of pre-clinical work, carried out for peptide-based fungal vaccines please see Da Silva et al (2020).

Barriers (and solutions) to Aspergillus vaccine development are discussed by Levitz (2017)