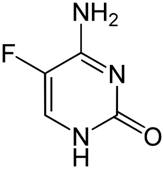
While most antifungals belong to three main categories (azoles, echinocandins, polyenes), there are several with other structures and mechanisms.
Flucytosine is on the WHO essential medicines list.

Factsheets
Flucytosine
| Names Flucytosine (5FC, 5-fluorocytosine); Ancotil (Ancobon) |
| STRUCTURE & MECHANISM A fluorinated pyrimidine analogue that was developed in 1957 at Roche Laboratories. Flucytosine is metabolised in fungal cells via the pyrimidine salvage pathway, where it acts as a subversive substrate. Metabolism produces toxic nucleotides that interfere with nucleic acid and protein synthesis. After transport into the fungal cell it is converted to 5-fluorouracil (5-FU) by the protein cytosine deaminase and then subsequent metabolites inhibit the enzyme thymidylate synthetase. Reduced thymidine leads to reduced DNA synthesis. Mammalian cells do not contain cytosine deaminase. |
| FORMULATIONS & DOSAGES Available as tablets (500 and 250 mg) and as an IV formulation (2.5 g of flucytosine in 250 ml normal saline). |
| ACTIVITY – ACTIVE against yeasts Cryptococcus neoformans and Candida spp., although Candida krusei and C. neoformans have higher MICs than other species. Occasional outbreaks of resistant isolates of Candida spp. have been described. Flucytosine has some anti-Aspergillus activity, with uncertainty about the rapidity of development of resistance. Flucytosine is active against Phialophoraspp., Cladosporium spp. and Exophiala spp. – NOT ACTIVE against Fusarium spp., Mucorales spp., Madurella spp., Microsporum spp., Trichophyton spp., Epidermophyton spp., Blastomyces dermatitidis, Paracoccidioides brasiliensis, Histoplasma capsulatum and Coccidioides immitis. |
| TYPICAL REGIMEN The standard dose of flucytosine is 100–150 mg/kg per day, divided between four doses (i.e. 37.5 mg/kg every 6 hours), unless there is reduced renal function. Significantly lower dosages may be as effective and are being studied. It is usually given with amphotericin B in the treatment of cryptococcal meningitis, and less often for invasive candidiasis or invasive aspergillosis. If creatinine clearance is >40 ml/minute, standard dosage can be used; if 20–40 ml/min, the same dose given 12-hourly is appropriate; if <20 ml/min, once daily 37.5 mg/kg dose can be given. Flucytosine is removed by haemodialysis and optimal serum levels of flucytosine can be achieved with single doses of 25–30 mg/kg body weight after each dialysis. Neonates on standard doses tend to have higher levels than adults for unclear reasons. |
| PHARMACOKINETICS Flucytosine is excreted almost entirely by glomerular filtration, and dose reduction is necessary with renal dysfunction (see above). Various algorithms are available to ensure dosage reduction results in effective and nontoxic drug levels. As it is highly water soluble, it gets into urine, CSF, peritoneal fluid, cardiac vegetations and the eyes in good concentrations. |
| SIDE EFFECTS & TOXICITY Oral flucytosine is generally well tolerated, but occasionally causes nausea and diarrhoea. Sometimes more severe gastrointestinal symptoms occur. Skin rashes have occasionally been observed, including a photosensitive rash. Flucytosine suppresses bone marrow function probably via the production of its metabolite and has been reported in 6–22% of patients. Myelosuppression is more likely with elevated flucytosine serum levels. Transient hepatomegaly and elevated transaminase levels are occasionally seen, especially if there are sustained high serum levels. Flucytosine is teratogenic at concentrations lower than those used for cryptococcal meningitis and should be avoided, especially during early pregnancy. |

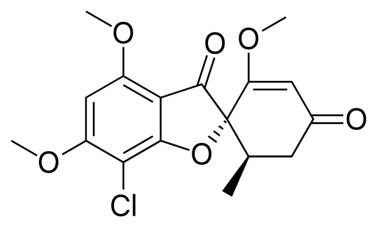
Griseofulvin
| NAMES Griseofulvin |
| STRUCTURE & MECHANISM A metabolite of Penicillium griseofulvum and Penicillium janczewskii. It binds to and disrupts both the α and β tubulin subunits inducing conformational changes which disrupts the fungal mitotic spindle. As microtubules are involved in the transport of secretory material through the cytoplasm this has a profound cellular effect. As a consequence, griseofulvin is a potent mutagen in multiple mammalian cell test assays. |
| FORMULATIONS & DOSAGES Griseofulvin is formulated as both microsize and ultramicrosize preparations given orally. In adults, the dose of the microsize preparation is 0.5–1 g daily, administered once or twice daily. Microsize griseofulvin should be administrated with a fatty food such as cheese or other dairy product, chocolate, fried food or nuts. The dosages of the ultramicrosize preparation are 330–660 mg daily. In children, the dose of microsize griseofulvin is 10 mg/kg daily and for the ultramicrosize preparation 5.5 mg/kg daily. |
| ACTIVITY – ACTIVE against the dermatophytes Microsporum spp., Trichophyton spp. and Epidermophyton spp. are susceptible to griseofulvin. – NOT ACTIVE against Candida spp., Aspergillus spp., Fusarium spp., Mucorales spp., Blastomyces dermatitidis, Paracoccidioides brasiliensis, Histoplasma capsulatum or Coccidioides immitis. |
| TYPICAL REGIMEN In children with tinea capitis, oral griseofulvin 500 mg/day had an efficacy of 88% over 6 weeks (Lipozencic et al, 2002). For tinea corporis topical therapy with griseofulvin may be effective (less than terbinafine of itraconazole), and oral therapy, 500 mg daily for 3-6 weeks, results in a 60% response rate. Griseofulvin is relatively ineffective for onychomycosis and not recommended. Griseofulvin may be effective at the elevated dose of 500 mg three times daily for 12 months for eumycetoma caused by Madurella spp. |
| PHARMACOKINETICS Griseofulvin reduces the anticoagulant effect of warfarin and ciclosporin levels, probably through CYP P450 enzyme induction. Griseofulvin may impair the performance of skilled tasks (e.g. driving) and enhances the effects of alcohol. Griseofulvin administration leads to accelerated oestrogen and progestogen metabolism, possibly reducing the efficacy of contraceptives. Griseofulvin reduces serum salicylate concentrations. |
| SIDE EFFECTS & TOXICITY Griseofulvin is sometimes not well tolerated. The most common side-effects are nausea, vomiting, diarrhoea, heartburn, flatulence, cracking at the side of the mouth, soreness and/or blackening of the tongue and thirst. Headache is a frequent side-effect (<15%), but may resolve on continued therapy. Other neurological side effects such as peripheral neuropathy may occur. Maculopapular, urticarial or photosensitivity rashes occasionally occur. Cutaneous lupus erythematosus lesions occurred in several patients with circulating antibodies to SSA/Ro and SSB/La autoantigens (nuclear and cytoplasmic polypeptides), and is contraindicated in systemic lupus erythematosus (SLE). Hepatotoxicity is infrequent but pre-existing liver disease may be exacerbated by griseofulvin. Griseofulvin is contraindicated in pregnancy (class C). As griseofulvin induces abnormalities in murine sperm, fertile men should be advised not to father children during therapy or for six months after the drug is stopped. |

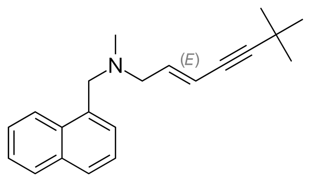
Terbinafine
| NAMES Terbinafine; Lamisil (Novartis) |
| APPROVAL 1992. Clinical studies commenced in the late 1980s and it is now off patent. It is the leading compound for fungal skin and nail infections. |
| A synthetic allylamine. It inhibits squalene epoxidase, one of the enzymes in the synthetic pathway of ergosterol, which is the major sterol in cell membranes (unlike in humans in which it is cholesterol). |
| FORMULATIONS & DOSAGES Oral and topical only. The standard dose is 250 mg daily. |
| ACTIVITY – ACTIVE against all the skin fungi, including Epidermophyton floccosum, Microsporum spp. and Trichophyton spp. It is also active against Malassezia furfur which causes pityriasis versicolor (tinea versicolor). It is active against most Candida species, although it may not be fungicidal in some species such as Candida albicans. There is some activity demonstrated against Aspergillus, but in combination with amphotericin B was antagonistic in invasive aspergillosis. – LIMITED ACTIVITY against other organisms. |
| TYPICAL REGIMEN The usual dose is 250 mg per day. This should be reduced to 50% in those with impaired renal function, and avoided in those with liver disease. Significant skin infections are treated for 3-4 weeks. Nail infections of the fingers require at least 6 weeks of treatment, but toenails require 3-6 months of therapy, depending on the extent of nail involvement. In the treatment of athletes foot, terbinafine cream can be applied between the toes daily for 7 days. |
| PHARMACOKINETICS Terbinafine is well absorbed when taken orally, and there is no effect of food on absorption. As it is a fat soluble drug it accumulates in skin, fatty tissue and nails. It persists in nails for long periods after the end of treatment. It is metabolised by the liver with a half life of 17 hours. This is prolonged in patients with liver or kidney impairment. |
| DRUG INTERACTIONS Terbinafine has the advantage over the azoles in not affecting the metabolism of such drugs as cyclosporin and oral diabetic drugs. Its blood levels are reduced if given with rifampicin (rifampin) as this increases the rate of metabolism. Cimetidine, the anti ulcer drug, also inhibits its metabolism, so higher blood levels are found if the drugs are given together. Terbinafine inhibits CYP2D6 and so may increase levels of tricyclic antidepressants, β-blockers, SSRIs and monoamine oxidase inhibitors type B. Warfarin may need adjustment if given with terbinafine |
| SIDE EFFECTS & TOXICITY Terbinafine is well tolerated. Its common side effects are nausea, mild abdominal discomfort and headache. Allergic skin reactions have been reported, although these are uncommon. Loss of taste or altered taste is also reported. Rarely has liver dysfunction been reported. |