Candida (phylum Ascomycota) are yeasts that often live harmlessly as part of the body’s natural flora but can cause disease if they enter sterile sites (e.g. bloodstream, eyes) or overgrow on mucosa (e.g. oral thrush, VVC). Identification and susceptibility testing are crucial in order to select an antifungal that will be effective. In part this is because different species have varying ability to form biofilms on indwelling devices that form a barrier to penetration of antifungals (Cavalheiro & Teixeira, 2018).
- Jump to: Candida albicans, Candida auris, Candida glabrata, Candida krusei, Candida parapsilosis, Candida tropicalis
In recent years Candida auris has emerged as a cause of healthcare-associated bloodstream infections, which can be challenging to identify and is resistant to fluconazole. Report cases or get help with identification and infection control at CDC.gov. Read more about MALDI-ToF detection (Vatanshenassan et al, 2019), infection control (Caceres et al, 2019) or a review (Jeffery-Smith et al, 2018).
Many alternative therapists promote the ‘Candida diet’ as a cure for nonspecific symptoms such as fatigue, headaches and brain fog, but this is not supported by science. The diet is likely to make patients feel healthier as it is low in processed foods such as sugar and refined flour, rather than any effect on the growth of yeast in the gut. Read more at the Mayo Clinic

Clinical lectures & video lab protocols
Click here to download the slide decks for the lectures

Factsheets
Candida albicans
| NAMES Candida albicans (AKA Candida stellatoidea) Read a review: Dadar et al (2018) |
| NATURAL HABITAT Normal flora on mucous membranes and in the digestive tract of humans and animals. If isolated in the environment, this usually reflects contamination by human or animal excreta. |
| GEOGRAPHY Worldwide |
| PREVALENCE The most common cause of all mucosal and systemic/invasive forms of candidiasis. |
| DISEASES Causes the widest range of infections of any yeast, from superficial to systemic/disseminated infections: onychomycosis, vaginitis, oral thrush, oesophageal candidiasis, bloodstream infection (candidaemia), disseminated infections involving any deep organ as liver, kidney, lung, etc., meningitis, endocarditis, pericarditis, peritonitis, arthritis, osteomyelitis, endophthalmitis, etc. Deep and disseminated infections are more frequent in immunocompromised patients. In addition, causes chronic mucocutaneous candidiasis in patients with a genetic disorder of T cells. |
| CULTURE The colour of the colonies is cream, glistening, and sometimes waxy. The appearance is soft and the surface is smooth although some strains can became wrinkled with a mycelial border. Like Candida dubliniensis, which it is closely related to, it forms hyphae in liquid culture in the presence of serum. This forms the basis of the germ tube identification test. It forms biofilms and can mate. Mating requires switching between “white” and “opaque” colony morphologies. White cells are round and form shiny, domed-shaped colonies in contrast to opaque cells which are a little elongated and form darker, flatter colonies. Biosafety level 2 |
| ANTIFUNGAL RESISTANCE Most isolates of C. albicans are fully susceptible to all clinical antifungal drugs. Resistance to amphotericin B is very rare, whereas resistance to fluconazole and other azoles occurs in up to 7% of wild-type isolates. Increased rates of resistance are seen in AIDS patients taking low dose or intermittent fluconazole. Resistance to flucytosine may occur after exposure. Echinocandin resistance is rare and associated with prolonged therapy. |
| INDUSTRIAL USES Possibly removal of textile dyes prior to discharge of industrial effluents. One strain (TL3) is able to degrade phenol compounds and formaldehyde (Tsai et al, 2005). |

Typical colonies of C. albicans 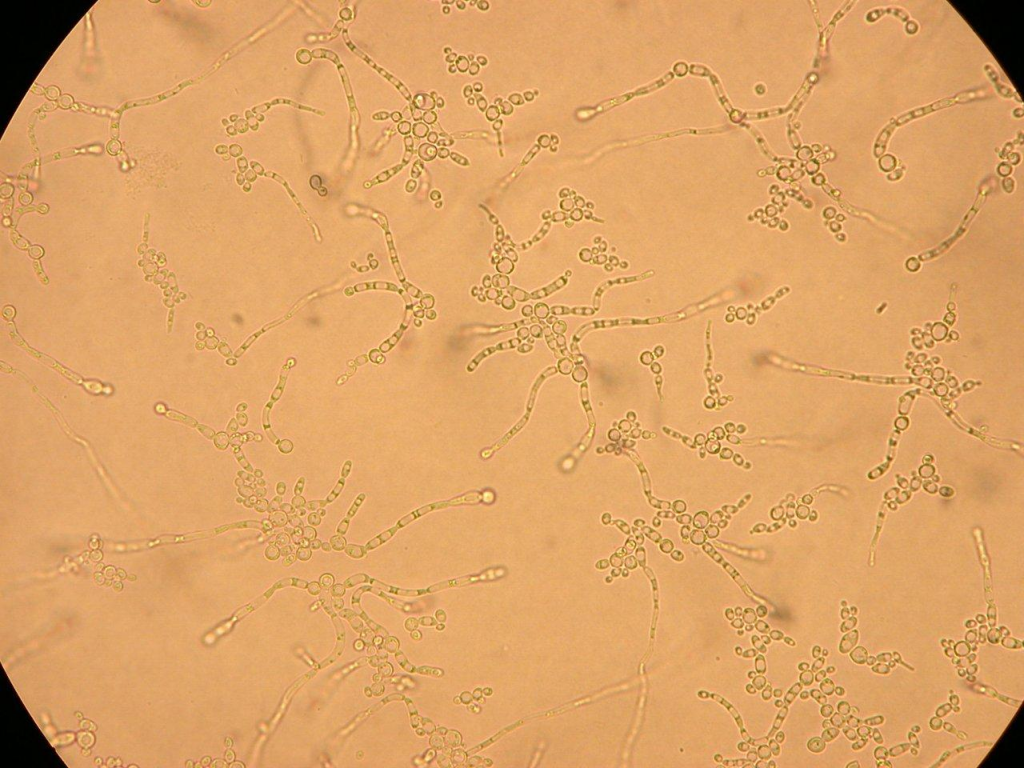
Microscopic appearance of C. albicans on corn meal agar 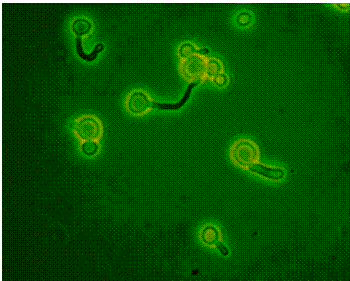
C. albicans germ tubes are useful in identification 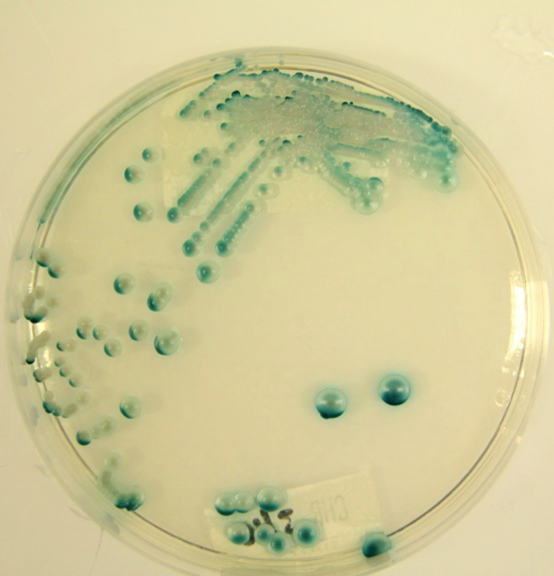
C. albicans grown on CHROMAgar™

Candida auris
| NAMES Candida auris The proposed taxonomic description of Candida auris sp. nov. is type strain JCM15448T= CBS10913T= DSM21092T. |
| NATURAL HABITAT So far, no environmental reservoir of C. auris has been identified. A significant percentage of genes in C. auris are devoted to central metabolism, a property that is common to pathogenic Candida spp. and crucial for adaptation in diverse environments. |
| GEOGRAPHY Four distinct clades of clinical isolates have been identified from separate geographic origins on three continents, suggesting a recent and nearly simultaneous emergence of different clonal populations. Several geographically related clusters have been reported from South Korea, India, South Africa, Pakistan, and hospitals in Latin America. Clonality within C. auris has been shown using AFLP, MLST and MALDI-ToF MS among strains in India, South Africa, and Brazil. Next generation sequencing (NGS) has demonstrated highly-related C. auris isolates in four unrelated and geographically separated Indian hospitals, suggesting that each clade exhibits low diversity. |
| PREVALENCE Candida auris is an emerging pathogen, first reported in 2009 from an ear canal infection in Japan. More recently, it has been associated with life-threatening invasive diseases such as bloodstream and wound infections globally. In a span of only seven years, this yeast which displays clonal inter- and intra-hospital transmission, has become widespread across several countries, causing a broad range of healthcare-associated invasive infections. The increasing isolation of C. auris from various clinical specimens clearly indicates its ability to colonise, invade and cause disease with varying severity. |
| DISEASES Candida auris has been reported to cause bloodstream infections, wound infections, and otitis. It has also been cultured from urine and the respiratory tract; however, whether isolation from these sites represents infection versus colonisation in each instance is unknown. C. auris has been recovered in samples from blood, catheter tips, CSF, bone, ear discharge, pancreatic fluid, pericardial fluid, peritoneal fluid, pleural fluid, respiratory secretions (including sputum and bronchoalveolar lavage), skin and soft tissue samples (both tissue and swab cultures), urine, and vaginal secretions. Clinically, it has been implicated as a causative agent in fungaemia, ventriculitis, osteomyelitis, malignant otitis (including otomastoiditis), complicated intra-abdominal infections, pericarditis, complicated pleural effusions, and vulvovaginitis. Much like other Candida species, there is uncertainty about the ability of C. auris to cause true respiratory, urinary, and skin and soft tissue infections despite being isolated from such samples. While one study reported no C. auris attributable deaths among nine patients with fungaemia, crude mortality rates for C. auris fungaemia have otherwise ranged from 28-66% across a wide range of healthcare settings and patient populations. |
| CULTURE C. auris is indistinguishable from most other Candida species by microscopy . C. auris grows readily at 37–42 °C and forms light pink colonies on chromogenic agars. Differentiation of C. auris from C. haemulonii complex using CHROMagar Candida supplemented with Pal’s medium. Unlike C. haemulonii, C. auris does not form pseudohyphae however some strains can form rudimentary pseudohyphae on cornmeal agar. C. auris is capable of forming biofilms and adhering to catheter material, although not to the same degree as Candida albicans. Biosafety level 2 |
| IDENTIFICATION C. auris is phylogenetically related to Candida haemulonii and Candida ruelliae. Previously and currently mis-identified as Candida duobushaemulonii, C. haemulonii, C. famata, C. krusei, C. lusitaniae, C. sake, Saccharomyces spp., Rhodotorula glutinis or Kodamaea ohmeri by commercial identification systems, such as Vitek 2 and bioMérieux Industry API®/ID32. The CDC recommends further testing for C. auris whenever C. haemulonii is identified or in a number of other scenarios depending on the organism reported and the method of identification. Accurate identification can be performed with VITEK MS and Bruker Biotyper matrix-assisted laser desorption/ionization time-of-flight (MALDI-ToF) devices using their ‘research use only’ databases. – More information on the CDC website Molecular sequencing of the D1–D2 domain of the 28S ribosomal DNA can also identify C. auris. – Download the PCR protocol In the U.S., all confirmed isolates of C. auris should be reported to local and state public health officials and CDC at candidaauris@cdc.gov |
| ANTIFUNGAL RESISTANCE Almost all strains of C. auris are resistant to fluconazole and many are resistant to amphotericin B, voriconazole, echinocandins and flucytosine. There are no Clinical and Laboratory Standards Institute (CLSI) or European Committee for Antimicrobial Susceptibility Testing (EUCAST) defined breakpoints for C. auris susceptibility. Fluconazole (tentative MIC breakpoint ≥32) is associated with high minimum inhibitory concentrations (MICs) with MIC50 results between 64–128 mg/L and MIC90 results between 64–256 mg/L by CLSI microbroth dilution, in four studies. Echinocandins (tentative MIC breakpoint ≥4 for anidulafungin and micafungin, ≥2 for caspofungin) appear to be most active in these studies with favorable results for anidulafungin (MIC50 range 0.125–0.5, MIC90 range 0.5–1), caspofungin (MIC50 0.25–0.5, MIC90 1), and micafungin (MIC50 0.125–0.25, MIC90 0.25–2). Amphotericin B (tentative MIC breakpoint ≥2) susceptibility testing exhibits a wider range of MIC results (MIC50 0.5–1, MIC90 2–4) and is likely less reliable as empiric therapy. In comparison to CLSI microbroth dilution, similar MIC50 and MIC90 results can likely be obtained by the EUCAST method. Caution should be used when interpreting Etest and Vitek antifungal susceptibility testing results. |
| INDUSTRIAL USES None |

Candida glabrata
| NAMES Candida glabrata (Nakaseomyces glabrata, previously Torulopsis glabrata) [teleomorph: none] Reviewed by Rodrigues et al (2013) |
| NATURAL HABITAT It is a saprophyte of the human being. It has also been found in baker´s yeast, excreta of cossidae larvae, birch wood, orange and passion fruit juice. |
| GEOGRAPHY Worldwide |
| PREVALENCE Depending on the geographical localization, C. glabrata is the second commonest cause of mucosal candidiasis (especially recurrent Candida vaginitis after multiple azole treatments) and a frequent cause of candidaemia and invasive yeast infections. |
| DISEASES It is the second most common cause of vaginitis. It is frequently isolated in cases of oropharyngeal and oesophageal candidiasis in AIDS patients, candidaemia and urinary tract infections. It is also able to produce deep and disseminated infections especially in immunosuppressed patients. Produce biofilms. |
| CULTURE The colonies in culture media are cream coloured. The appearance is soft and smooth and the surface is glossy. C. glabrata does not produce hyphae, pseudohyphae or chlamydospores. It reproduces by means of budding. Biosafety level 2 |
| ANTIFUNGAL RESISTANCE Most strains are susceptible to amphotericin B, flucytosine and echinocandins. C. glabrata easily develops secondary resistance to azole drugs especially to fluconazole so this group of antifungals should be avoided in the treatment of infections caused by this microorganism. One exception may be urinary tract infections when fluconazole is highly concentrated in urine. |
| INDUSTRIAL USES It has been used as a biosurfactant. |
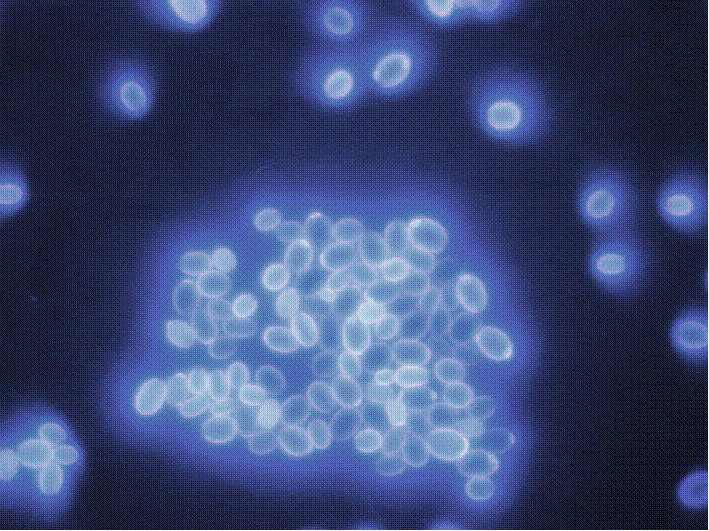
Microscopical appearance of C. glabrata 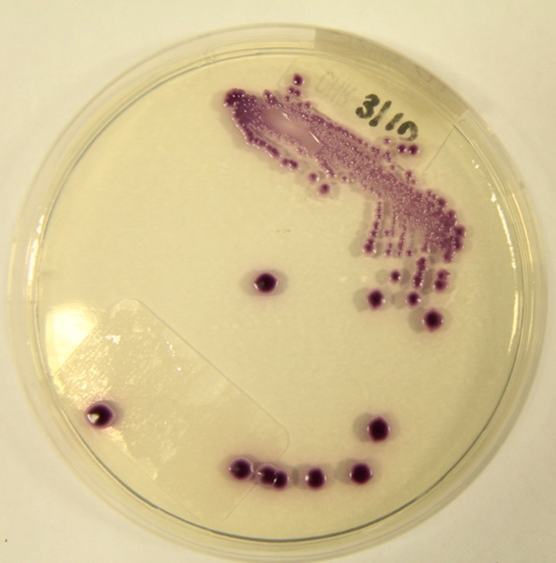
C. glabrata grown on CHROMAgar™

Candida parapsilosis
| NAMES Candida parapsilosis The Candida parapsilosis complex is divided into three groups/species on the basis of molecular analysis: – C. parapsilosis (92% of isolates) – C. orthopsilosis (6%) – C. metapsilosis (2%) Reviewed by van Asbeck et al (2009). |
| NATURAL HABITAT Insects, soil, domestic animals, marine environments. It is a commensal member of the human microbiome; isolated from mucosal surfaces, skin and nails. Frequently isolated from physical surfaces in hospital environments |
| GEOGRAPHY Worldwide |
| PREVALENCE Varies with geographical region; blood culture yield 12-20% of all Candida spp. Second or third most common Candida species after C. albicans causing candidaemia. |
| DISEASES Invasive candidiasis, usually in non-immunocompromised patients. Catheter and intravenous line infections (often forming biofilms). Associated with an extended hospital stay. Rare cause of primary mucosal infection such as vaginal candidosis. May cause urinary tract infection, endocarditis, peritonitis, joint infection and meningitis. Very rare cause of endophthalmitis, in contrast to C. albicans. |
| CULTURE Pin-like colonies on CHROMagar. Appearance on Columbia Horse Blood Agar or Sabouraud is white to cream, and smooth in texture. Biosafety level 2 |
| ANTIFUNGAL RESISTANCE Usually fully susceptible, with C. parapsilosis (but not C. orthopsilosis nor C. metapsilosis) being less susceptible to caspofungin, micafungin and anidulafungin. Occasional acquired resistance to all azole drugs with no inherent/intrinsic resistance to azoles noted in literature. Biofilm formation is a significant factor in multidrug resistance. Possibly not killed effectively by amphotericin B. |
| INDUSTRIAL USES Stereospecific biosynthesis of chiral alcohols, xylitol and xylulose production. |
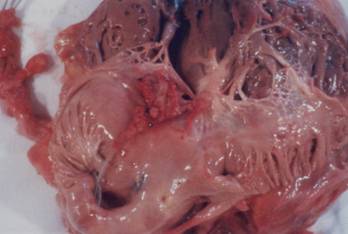
C. parapilosis infecting pacemaker leads 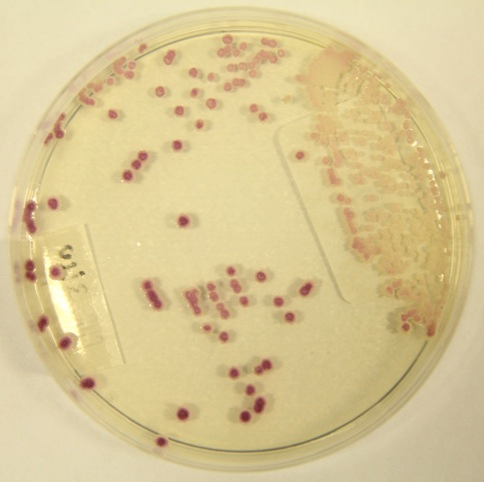
C. parapsilosis grown on CHROMAgar™
