Antibody tests detect a patient’s immune response against a pathogen, which can show whether they have been exposed or sensitised to a pathogen, or whether they are responding well to treatment. Clinical applications are well established for a number of fungal conditions:
- Diagnosis/monitoring of treatment of coccidioidomycosis
- Diagnosis/monitoring of treatment of chronic pulmonary aspergillosis
- Screening for/management of allergic bronchopulmonary aspergillosis (ABPA)
- Diagnosis/monitoring of treatment of allergic, chronic and granulomatous Aspergillus rhinosinusitis
- Supportive information for the diagnosis of Aspergillus bronchitis in non-immunocompromised patients.
- Diagnosis of acute (seroconversion) and chronic histoplasmosis
- Diagnosis of paracoccidioidomycosis
- Diagnosis and monitoring of candidiasis
Other antibody tests are also available with limited utility in clinical practice.
- Anti-Pneumocystis and anti-Cryptococcus antibody tests have been useful in research studies to document rates of prior exposure and infection, especially in children.
- Some have been combined with antigen testing for diagnostic purposes, e.g. anti-Candida mannan antibodies.
- Anti-Sporothrix antibodies have been detected in CSF during rare cases of Sporothrix meningitis,
- Anti-Scedosporium antibodies have been used to diagnose fungal ball due to S. apiospermum, or distinguish causative agents in mycetoma

Types of antibody test
TEST FORMATS: several test formats are used for the detection of antibody in blood including: double-diffusion (DD); immunodiffusion (ID); counterimmunoelectophoresis (CIE); enzyme-linked immunosorbent assay (ELISA); complement fixation (CF). Less commonly used methods include: haemagglutination; radioimmunoassay; immunoblotting; co-CIE. The relative strengths and weaknesses of these methods are described in textbooks and are not reviewed here.
IMMUNOGLOBULIN TYPE: Almost all tests detect IgG or IgE antibodies, with the exception of IgM for coccidioidomycosis. No utility has been found for the detection of IgA antibodies to fungi. It is possible that IgM antibodies might have more clinical utility than currently realised, especially for aspergillosis, but are not routinely available.

Videos & images




Factsheets
Coccidioides
| OVERVIEW Antibody detection is key to the diagnosis of several manifestations of coccidioidomycosis. Early (IgM) and late (IgG) antibodies may be produced. – IgM becomes detectable usually between the first (~50%) and third (~90%) weeks after symptoms in primary coccidioidal infection, except in the immunocompromised patient. – IgG becomes detectable between the second and 28th week post onset of illness. |
| AVAILABLE ASSAYS – EIA: Meridian; MVista Coccidioides Antibody IgG IgM EIA – POCT: IMMY sona™ rapid (<30 mins) LFD for both IgG and IgM (sensitivity ~100%; specificity ~77%) |
| DIAGNOSIS – Coccidioidal meningitis: the earliest specific feature is IgM antibody in CSF. As microscopy and culture are usually negative, and the cell count, protein and glucose are consistent with tuberculous meningitis, the diagnosis can be missed. |
| MONITORING TREATMENT Serial titration of CF antibody is useful in the evaluation of improvement of worsening of disease, and in evaluation of efficacy of antifungal therapy. Parallel testing of previously collected and new specimens may be required to ensure consistency. Increasing CF titers suggest worsening or dissemination; decreasing titers indicate therapeutic response. |
| PERFORMANCE & INTERPRETATION Negative serologic results cannot be used to rule out coccidioidomycosis (particularly early in the acute phase). The overall sensitivity of anticoccidioidal serologic studies is ~82%. ELISAs for detection of both IgM and IgG are probably the most sensitive of the methods, compared with tube precipitins for IgM and CF for IgG, but may be less specific. |

Aspergillus
| OVERVIEW IgG testing of serum is a mainstay in the diagnosis of chronic pulmonary aspergillosis, while elevated total serum levels of IgE is diagnostic of allergic bronchopulmonary aspergillosis. |
| AVAILABLE ASSAYS – A. fumigatus IgG: Immy, Serion/Virion, Bioenche, BioRad, Thermofisher, Elitech, Microgen, NovaTec and Bordier. Lateral flow devices also available from LDBio and Era Biology. A. fumigatus IgG assay is also available using the ELISA method, from abcam Detecting allergen-specific IgE antibodies in human serum using the IMMULITE Specific IgE assay is available with Siemens. |
| DIAGNOSIS SPECIES: There are multiple marketed IgG antibody tests to detect A. fumigatus antibodies. A smaller number of less well used and often usually incompletely validated tests are available for other species of Aspergillus including A. flavus, A. terreus, A. niger, A. versicolor and A. clavatus. SAMPLE: All testing is on serum. DETECTING SENSITISATION: IgE antibody testing against A. fumigatus is useful to detect Aspergillus sensitisation. The skin prick test against A. fumigatus is more sensitive than blood testing. Either is required for the diagnosis of ABPA (usually both are positive) and are usually positive at a much lower level in patients with severe asthma with fungal sensitisation (SAFS). This test could be a useful screening test in asthmatic patients to detect ABPA or SAFS, if skin testing not done (IgE measurement) CPA: Some patients with other Aspergillus diseases, notably chronic pulmonary aspergillosis, have positive Aspergillus IgE antibody titres. Occasionally this test is positive when the IgG antibody test is negative, which is helpful (view Skin testing and IgE testing) |
| MONITORING TREATMENT Falling IgG antibody titre is useful as a measure of therapeutic response in chronic pulmonary aspergillosis and Aspergillus rhinosinusitis. The rate of fall is slow and takes weeks or months even in patients doing well. Lack of fall is suspicious of treatment failure. |
| PERFORMANCE & INTERPRETATION The best IgG assays have a 90-95% sensitivity for chronic pulmonary aspergillosis and aspergilloma caused by A. fumigatus, much more sensitive than culture. Requires compatible radiology and relatively non-immunocompromised patient for the diagnosis to be made. The antibody titre varies widely. 30-50% of patients with ABPA have detectable A. fumigatus antibodies, usually at low titre. High titres suggest the complication of chronic pulmonary aspergillosis, which needs radiological correlation. Fungal sinusitis is more often caused by A. flavus than A. fumigatus. A. flavus IgG antibody is useful confirmatory evidence of infection, especially if cultures are negative but histology or microscopy positive. Patients with Aspergillus bronchitis often have positive IgG antibodies (and negative IgE antibodies) but A. fumigatus is slightly less common as the cause, so non-fumigatus IgG antibodies (precipitins) may be required. The optimum cut-off for positive IgG titres varies and may be higher than some manufacturers recommend, because healthy control sera (which almost always have very low antibody titres) may have a low titre in which is too low in specific groups. An example is cystic fibrosis in which the baseline Aspergillus IgG antibody titres are higher than normal controls. |

Candida
| OVERVIEW Antibodies to Candida species, both agglutinins and precipitins, have been found in patients with and without systemic candidiasis. There are biochemical differences between the mycelial and blastospore forms of Candida albicans (Chattaway et al., 1968). The antigenicity of the two growth phases of Candida albicans differ in qualitative and quantitative ways with a specific mycelial antigenic component having diagnostic potential (Evans et al., 1973). Specific mycelial antigens are superior to yeast cell antigens in the diagnosis of suspected systemic candidosis (Umenai & Chiba 1977; Syverson, Buckley & Campbell 1975). These and later data (Peman et al., 2011) lead to the commercialisation of a Candida albicans indirect chemiluminescent immunoassay for the detection of antibodies against antigens located on the cell wall surface of the mycelium of Candida albicans (CAGTA [Candida albicans germ tube antibody]) in human serum or plasma (Vircell, Spain). |
| AVAILABLE ASSAYS Platelia™ Candida Ab Era Biology Candida Ab Dynamiker Candida Ab GaDia CandiDia Era Biology aslso have an Mannan IgM antibody detection test. |
| DIAGNOSIS |
| MONITORING TREATMENT |
| PERFORMANCE & INTERPRETATION The test appears to discriminate between infection and colonization and it has a high negative predictive value (73%). Combining the CAGTA assay with the ß-1-3-D-glucan test (Fungitell, Associates of Cape Cod, USA) in patients on empirical antifungal therapy for presumed candidiasis has shown that serial determination of CAGTA and glucan during empirical antifungal therapy has a high sensitivity (93%) and negative predictive value (91-97%). If properly confirmed, this strategy could be used to discontinue antifungal treatment in at least 30% of patients as a complementary tool in antifungal stewardship programmes (Martinez-Jiménez et al., 2015; supplementary data). |

Histoplasma
| OVERVIEW Antibody detection is useful for the chronic forms of histoplasmosis, notably chronic pulmonary histoplasmosis and some patients with (subacute) progressive disseminated histoplasmosis. Seroconversion is often documented after acute pulmonary histoplasmosis. |
| AVAILABLE ASSAYS Antibody kits: Meridien; Immy; MiraVista (Available through MiraVista labs in Indiannapolis) |
| DIAGNOSIS Seroconversion from negative to positive is the most straightforward means of making a diagnosis of acute pulmonary histoplasmosis but it may take 2 to 6 weeks to seroconvert. Over 90% of patients with chronic pulmonary histoplasmosis have detectable antibodies to Histoplasma. About 30-70% of patients with disseminated histoplasmosis have detectable antibodies, depending on the immunological status of the host. |
| PERFORMANCE & INTERPRETATION Cross reaction is common with sera from patients with blastomycosis, penicilliosis and paracoccidioidomycosis and so the test cannot be used to differentiate these infections. Both complement fixation (CF) and immunodiffusion (ID) antibodies may be positive, without a clear advantage between them in sensitivity, but specificity appears to be higher with ID. If one test is negative the other may be positive. |

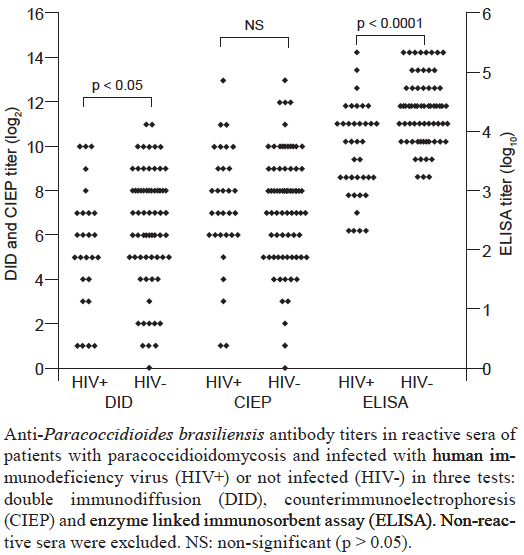
Paracoccidioides
| OVERVIEW Several serological assays detecting antibody have been used for the early diagnosis of Paracoccidioides brasiliensis infection. Standardisation has been difficult because of variations in assay sensitivity, specificity and reproducibility mostly related to lot to lot variations in the preparation of antigens required for the assays. The most frequently used test is double immunodiffusion assay which has a sensitivity and specificity ranging from ~80 to 90%. Antigens preparations containing high concentration of the 43 KDa glycoprotein are required to optimize performance of the test. |
| AVAILABLE ASSAYS Only available in reference and university hospital laboratories – no commercial test. |
| DIAGNOSIS |
| MONITORING TREATMENT Falling IgG antibody titres is useful as a measure of therapeutic response in acute and chronic forms of paracoccidioidomycosis. The rate of fall is slow and takes months or years even in patients doing well. Lack of fall in antibody titre is suspicious of treatment failure. |
| PERFORMANCE & INTERPRETATION ELISA has a higher sensitivity but false-positive results have been frequently reported in normal individuals living in endemic areas for P. brasiliensis as well as in patients with tuberculosis, leprosy, leishmaniasis and other medical conditions. In home made tests, the following results were obtained with 3 different test formats View paper These data are consistent with a useful role for antibody testing in acute and chronic forms of paracoccidioidomycosis in which sensitivity of different assays may exceed 90% . Despite the good results of serology obtained with patients co-infected with paracoccidioidomycosis and AIDS in this publication, other experienced laboratories describe limited sensitivity of antibody detection in the presence of comorbidities associated with immunodeficiency. |